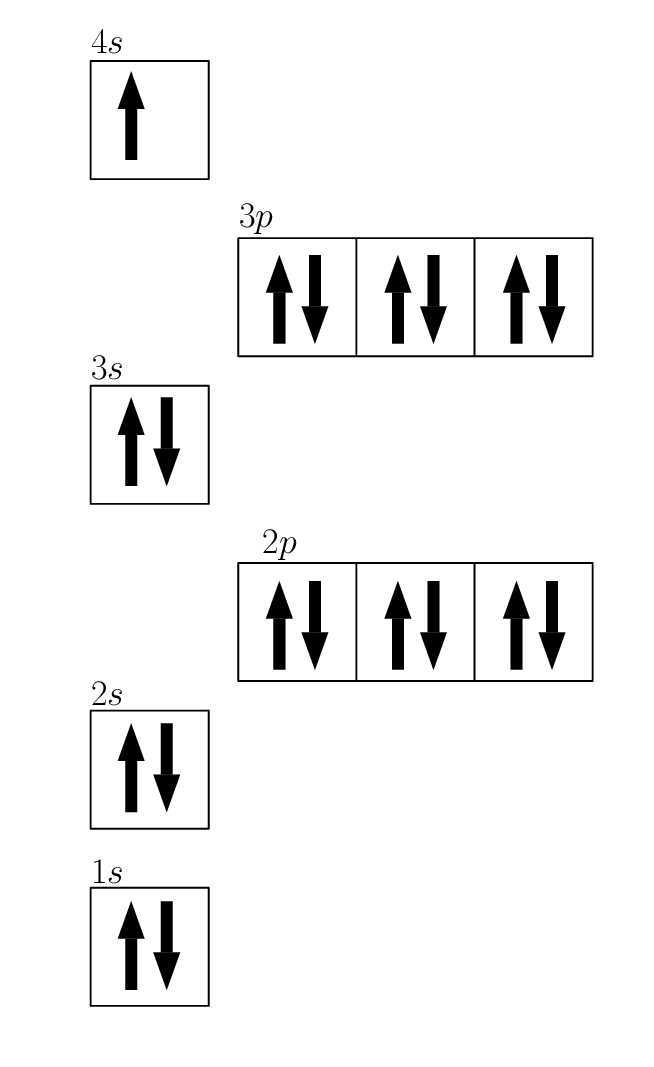
Neutral Iron Electron Configuration . The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its valence orbitals are the 4s and 3d's. It’s a transition metal from group 8 of the periodic table’s first transition series. Writing the electron configuration, you really only need the. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Commonly, the electron configuration is. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ;
from www.siyavula.com
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Writing the electron configuration, you really only need the. Commonly, the electron configuration is. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. Its valence orbitals are the 4s and 3d's. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.
4.6 Electronic configuration The atom Siyavula
Neutral Iron Electron Configuration It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. It’s a transition metal from group 8 of the periodic table’s first transition series. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Its valence orbitals are the 4s and 3d's. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Commonly, the electron configuration is. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; Writing the electron configuration, you really only need the.
From circuitlistaneurin123.z21.web.core.windows.net
Carbon Electron Configuration Full Neutral Iron Electron Configuration Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Writing the electron configuration, you really only need the. It’s a transition metal from group 8 of the periodic table’s first transition series. Its valence orbitals are the. Neutral Iron Electron Configuration.
From studylib.net
Writing Electron Configuration for Neutral Atoms Full Notation Core Neutral Iron Electron Configuration Writing the electron configuration, you really only need the. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding. Neutral Iron Electron Configuration.
From circuitlistaneurin123.z21.web.core.windows.net
Carbon Electron Configuration Full Neutral Iron Electron Configuration The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Commonly, the electron configuration is. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; Writing the electron configuration, you really only need. Neutral Iron Electron Configuration.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Neutral Iron Electron Configuration Its valence orbitals are the 4s and 3d's. Commonly, the electron configuration is. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. The electron configuration of iron, specifically fe2+. Neutral Iron Electron Configuration.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications Neutral Iron Electron Configuration Its valence orbitals are the 4s and 3d's. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. The configuration notation provides an easy way for scientists to write and communicate how electrons are. Neutral Iron Electron Configuration.
From fity.club
Iron Electron Configuration Neutral Iron Electron Configuration Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The chemical element iron has the atomic number 26 and the symbol fe (from. Neutral Iron Electron Configuration.
From circuitdiagramrusa.z21.web.core.windows.net
Determine The Electron Configuration Of Fe3+ Neutral Iron Electron Configuration Its valence orbitals are the 4s and 3d's. Commonly, the electron configuration is. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Writing the electron configuration, you really only need the. Its core orbitals are the 1s, 2s, 2p's, 3s,. Neutral Iron Electron Configuration.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry Neutral Iron Electron Configuration The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic. Neutral Iron Electron Configuration.
From material-properties.org
Iron Periodic Table and Atomic Properties Neutral Iron Electron Configuration Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. The configuration notation provides an easy way for scientists to write and communicate how electrons are. Neutral Iron Electron Configuration.
From mavink.com
Electron Configuration Chart With Orbitals Neutral Iron Electron Configuration Writing the electron configuration, you really only need the. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Its valence orbitals are the 4s and. Neutral Iron Electron Configuration.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry for Neutral Iron Electron Configuration The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus. Neutral Iron Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Iron Neutral Iron Electron Configuration Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. Commonly, the electron. Neutral Iron Electron Configuration.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Neutral Iron Electron Configuration Commonly, the electron configuration is. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Writing the electron configuration, you really only need. Neutral Iron Electron Configuration.
From www.siyavula.com
4.6 Electronic configuration The atom Siyavula Neutral Iron Electron Configuration Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. Its valence orbitals are the 4s and 3d's. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Commonly, the electron configuration is. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table. Neutral Iron Electron Configuration.
From whatsinsight.org
Electron Configuration for Iron (Fe) What's Insight Neutral Iron Electron Configuration The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. Its valence orbitals are the 4s and 3d's. Commonly, the electron configuration is. It’s a transition metal from group 8 of the periodic table’s first transition series. The configuration notation. Neutral Iron Electron Configuration.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry Neutral Iron Electron Configuration The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide ; The. Neutral Iron Electron Configuration.
From spalery.weebly.com
Periodic table valence electron definition spalery Neutral Iron Electron Configuration The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The configuration notation provides an easy way for scientists to write and. Neutral Iron Electron Configuration.
From chartdevelopment.blogspot.com
Draw The Electron Configuration For A Neutral Atom Of Manganese Neutral Iron Electron Configuration Its valence orbitals are the 4s and 3d's. Its core orbitals are the 1s, 2s, 2p's, 3s, and 3p's. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of iron, specifically fe2+ and fe3+ ions, plays a crucial role in understanding its chemical properties. Using the aufbau principle, the pauli exclusion. Neutral Iron Electron Configuration.